In the pharmaceutical industry, training failures rarely look dramatic at first.
A missed SOP acknowledgment. An outdated training record. A system that can’t clearly show who was trained, on what version, and when.
But during an audit, these gaps quickly turn into findings—sometimes warning letters, delayed approvals, or costly operational shutdowns.
That’s why a learning management system for pharmaceutical companies cannot be generic. It must be designed for regulated environments where training is inseparable from compliance, quality, and patient safety.
Quadric IT’s pharma LMS is built for exactly this reality—where training isn’t just delivered, but provable.
What Is an LMS in the Pharmaceutical Industry?
A Learning Management System (LMS) in the pharmaceutical industry is not simply a training portal. It is a validated, compliance-driven system used to manage, deliver, track, and document employee training in line with regulatory expectations.
Its primary purpose is to ensure that every employee—from manufacturing and quality teams to sales and medical staff—is properly trained, and that there is a complete, audit-ready record to prove it.
Unlike LMS platforms used in non-regulated industries, a pharmaceutical LMS must support:
- GMP, SOP, and regulatory training
- Role-based competency tracking
- Automated certification and retraining
- Secure, traceable audit trails for inspections
Regulators don’t ask whether training exists—they ask when it occurred, which version was used, and where the evidence is stored. A pharma LMS serves as the system of record that answers those questions instantly .
The Real Cost of Inadequate Pharma Training Systems
Many pharmaceutical organizations still rely on spreadsheets, email-based tracking, or generic LMS platforms not designed for compliance. Over time, this creates measurable risk.
Common consequences include:
- Audit delays caused by incomplete or fragmented training records
- Manual reconciliation work for QA and compliance teams
- Inconsistent SOP training across sites and departments
- Slower onboarding for GMP-critical roles
In real-world pharma operations, organizations that move to a centralized, validated LMS typically experience:
- 30–40% faster audit preparation
- Reduced SOP retraining cycles
- Faster time-to-productivity for new hires
- Lower compliance administration overhead
The difference is not more training—it’s better systems.
Types of LMS: Which One Works for Pharma & Life Sciences?
Not all LMS platforms are suitable for regulated environments. Understanding the differences is critical when selecting the right solution.
1. Generic or Corporate LMS
Designed for broad corporate learning, these platforms focus on employee development and soft skills. They often lack:
- Validation support
- GxP compliance features
- Audit-grade reporting
For pharmaceutical companies, these gaps quickly become liabilities.
2. Organizational LMS (Pharma-Specific)
An organizational LMS built for pharma focuses on compliance-first training. It supports:
- GMP and SOP management
- Role-based learning paths
- Certification and retraining automation
Quadric IT’s LMS pharma software belongs to this category—engineered to support pharmaceutical manufacturing, quality, R&D, and commercial operations.
3. Life Sciences & Bio LMS
These platforms extend beyond pharma to serve life sciences, biotechnology, and healthcare organizations. They manage:
- Clinical and laboratory training
- Multi-region regulatory requirements
- Complex learning needs across research and production
The LMS is flexible enough to support pharmaceutical, life sciences, and bio organizations, making it suitable for both focused and diversified enterprises.
Quadric IT’s LMS: Built for Regulated Pharma Environments
Quadric IT delivers an LMS software for pharmaceutical industry organizations that treats compliance as a system capability—not a manual process.
Key Capabilities
Automated Compliance & Audit Readiness
Training completion, certifications, and retraining cycles are automatically tracked. Detailed reports are available instantly, helping teams stay prepared long before an auditor arrives.
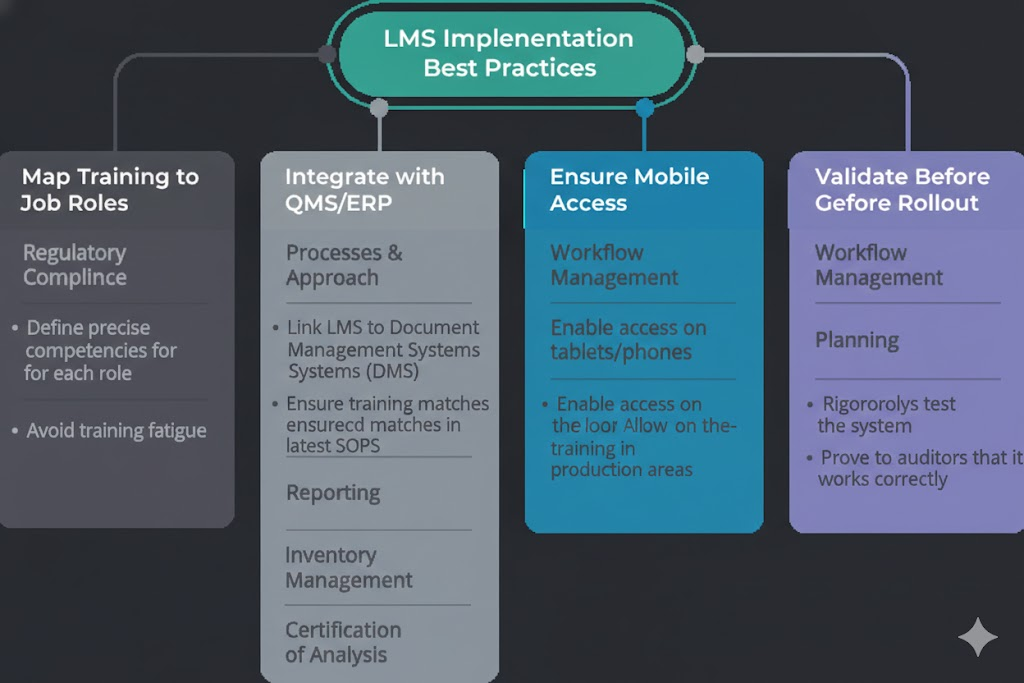
SOP & GMP Training Management
The system tracks SOP acknowledgments and ensures retraining is triggered automatically when procedures change—eliminating gaps caused by outdated documentation.
Role-Based Learning Paths
Training is tailored by role, department, and responsibility. Employees receive only what is relevant, improving efficiency without compromising compliance.
Engaging, Structured Learning Delivery
Support for videos, documents, assessments, and virtual sessions keeps training practical and effective, while remaining fully traceable.

ERP-Based LMS Customizations: Where Quadric IT Truly Differentiates
Most LMS platforms operate separately from core business systems. In pharmaceutical environments, this separation creates risk.
Quadric IT integrates learning directly into enterprise workflows through ERP-based LMS customizations, ensuring training is enforced where work actually happens.
SAP LMS Customization
By integrating LMS functionality with SAP, Quadric IT enables:
- Training-gated system access
- Alignment between quality events and learning requirements
- A single, defensible source of truth for audits
Zoho LMS Customization
With Zoho LMS integrations, organizations can:
- Automate role-based training assignments
- Sync HR, operations, and learning data
- Monitor workforce readiness in real time
AI Tools for Healthcare & Pharma Industry
Quadric IT also applies specialized AI to:
- Recommend role-specific learning paths
- Identify training gaps before they become audit findings
- Improve assessment accuracy and knowledge retention
This approach transforms the LMS from a passive repository into an active compliance enforcement layer.
Why Quadric IT’s LMS Is the Best Choice for Pharma Companies
Pharmaceutical companies don’t fail audits because they lack training. Ultimately, they fail when their systems can’t prove compliance at scale.
Quadric IT’s LMS is designed to solve that problem.
By combining:
- Pharma-grade compliance architecture
- ERP-integrated LMS customization (SAP, Zoho)
- AI-supported learning intelligence
- Scalability across pharma, life sciences, and bio sectors
Quadric IT delivers a platform that reduces regulatory risk, strengthens quality culture, and supports sustainable growth—without adding operational complexity.
This is not just another LMS.
It is a validated foundation for workforce competency and regulatory confidence.
Ready to Eliminate Training Risk from Your Compliance Equation?
If your organization is preparing for audits, expanding operations, or modernizing legacy systems, now is the time to rethink how training is managed.
To move forward, book a personalized demo with Quadric IT and see how our pharma-focused learning management system keeps your workforce audit-ready—every day, not just during inspections.

